English synonyms: c.i.37500;c.i.azoiccouplingcomponent1;c.i.developer5;ChemicalbookDeveloperA;DeveloperAMS;DeveloperBN;developera;developerams
CAS Number: 135-19-3
Molecular formula: C10H8O
Molecular weight: 144.17
EINECS number: 205-182-7
Related categories:
Intermediates;AromaticCompounds;ColorFormer&RelatedCompounds;Developer;Alphabetical;BioactiveSmallMolecules;BiochemicalsandReagents;BuildingBlocks;alcohol;MICROCIDIN;pigments;Fluorescent;Naphthalene;DyestuffIntermediates;Aromatics;DyestuffIntermediates;BioxyLabs;Fluortheescent;Organsis;Bigenology;OrganicChemical ;ParticlesandStains;pHSensitiveProbesandIndicators;
2-naphthol use and synthesis method
| Maximum allowable use of food additives and maximum allowable residue standards | Additive name | The name of the food that allows the use of this additive | Additive function | Maximum allowable usage (g/kg) | Maximum allowable residue (g/kg) |
| Acetonitrile | Surface-treated fresh fruits (citrus fruits only) | preservative | 0.1 | Residual amount ≤70mg/kg |
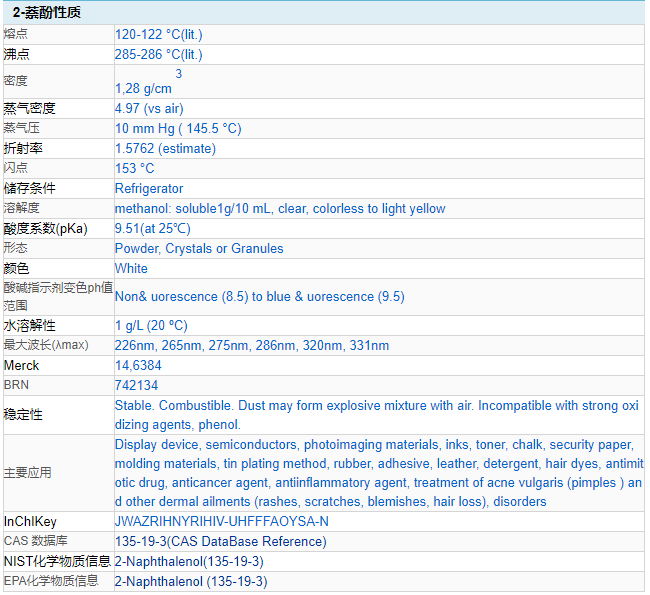
Chemical properties: white shiny flakes or white powder. Insoluble in water, soluble in ethanol, ether, chloroform, glycerin and alkali solutions.
Use:
1. It is used in the preparation of Tobias acid, J acid, 2,3 acid and azo dyes, and is also a raw material for rubber antioxidants, mineral processing agents, fungicides, antifungal agents, preservatives, etc.
2. As a preservative, my country stipulates that it can be used for citrus preservation, the maximum use amount is 0.1g/kg, and the residual amount is not more than 70mg/kg.
3. Ethylnaphthol, also known as β-naphthol and 2-naphthol, is an intermediate of the plant growth regulator naphthoxyacetic acid.
4. Used as a feed preservative. According to our country, it can also be used for citrus preservation. The maximum use amount is 0.1g/kg, and the residual amount is not more than 70mg/kg.
5. Used as analytical reagent, ethylene, carbon monoxide absorbent and fluorescent indicator
6. Important organic raw materials and dye intermediates, used in the manufacture of Toubic acid, butyric acid, β-naphthol-3-carboxylic acid, and used in the manufacture of anti-aging agent D, anti-aging agent DNP and other anti-aging agents, organic pigments and fungicides Wait.
7. Used in the preparation of Tobias acid, J acid, 2.3-acid, and used in the preparation of anti-aging agent D, anti-aging agent DNP and its anti-aging agents, organic pigments and fungicides
8. Verification of bromine, chlorine, chlorate, niobium, copper, nitrite and potassium. Fluorescence Chemicalbook photodetection substrate of phenolsulfonyltransferase. Qualitative determination of acid-base indicators, dyes, organic synthesis, allyl alcohol, methanol, chloroform, etc. Carbon monoxide, ethanol absorbent, fluorescent indicator.
9. Verification of bromine, chlorine, chlorate, niobium, copper, nitrite and potassium; determination of carbon monoxide, copper, nitrite and potassium; qualitative determination of allyl alcohol, methanol, chloroform, etc.; phenolsulfon transferase Fluorescence measurement substrate; ethylene absorber; fluorescent indicator; acid-base indicator; dye intermediate.
Production method:
1. It is made from naphthalene through sulfonation and alkali fusion. The sulfonated alkali fusion method is a widely used production method at home and abroad, but it has serious corrosion, high cost, and high biological oxygen consumption in wastewater. The 2-isopropylnaphthalene method developed by the American Cyanamide Company uses naphthalene and propylene as raw materials to produce 2-naphthol as a by-product of acetone. This method is similar to the production of phenol by the cumene method. Raw material consumption quota: refined naphthalene 1170kg/t, sulfuric acid 1080kg/t, solid caustic soda 700kg/t.
2.1) Sulfonated alkali melting method. Place the refined naphthalene in the sulfonation pot and heat (melt) to 140°C. Add 1.085 times (molar ratio) concentrated sulfuric acid within 20 minutes, increase the temperature, and keep it at 160-164°C for 2.5 hours. The reaction ends when the content of 2-naphthalenesulfonic acid reaches 66% or more and the total acidity is 25%-27%. The sulfonate is hydrolyzed in a hydrolysis pot at 140-150℃ for 1h. Then in a neutralization pot, neutralize with sodium hydrogen nitrite solution at 80-90°C until the Congo red test paper does not turn blue. Use steam and air to drive away the SO2 gas, cool to 30-40°C and then suction filter, wash with 10% salt water, and then suction filter to obtain sodium 2-naphthalenesulfonate. Place the sodium hydroxide in an alkali melting pot, heat (melt) to 290°C, and add sodium 2-naphthalenesulfonate in about 3 hours until the free alkali content is 5%-6%. Afterwards, it was kept at 320-330°C for 1 hour. Alkali melt is diluted in water and passed SO2 at 70-80°C until phenolphthalein is colorless. Add water to boil and wash, remove the sodium sulfite, and then dehydrate and distill under reduced pressure to obtain the finished product. The total yield is 73%-74%.
2) 2-isopropylnaphthalene method. Chemicalbook, which uses naphthalene and propylene as raw materials, reproduces 2-naphthol at the same time as a by-product of acetone.
3. The preparation method is to add sulfuric acid to molten naphthalene at 140°C, perform sulfonation at 162~164°C, hydrolyze the sulfonate, blow off the free naphthalene, and react with sodium sulfite to produce 2-naphthalenesulfonic acid sodium salt. The solid sodium salt and sodium hydroxide are alkali-melted at 285-320°C, and then kept at 320-330°C for 1 hour. The alkali melt is diluted and passed into sulfur dioxide for acidification to obtain a crude product, which is washed and dehydrated and then distilled to obtain the product.
4. Sulfonation alkali melting method Put refined naphthalene in a sulfonation pot and heat (melt) to 140°C. Add 1.085 times (molar ratio) concentrated sulfuric acid within 20min, increase the temperature, and keep it at 160~164℃ for 2.5h. The reaction ends when the content of 2-naphthalenesulfonic acid reaches 66% or more and the total acidity is 25%-27%. The sulfonate is hydrolyzed in a hydrolysis pot at 140~150℃ for 1h. Then in a neutralization pot, neutralize with sodium bisulfite solution at 80~90℃ until the Congo red test paper does not turn blue. Use steam and air to drive away the SO2 gas, cool to 30-40°C and then suction filter, wash with 10% salt water, and then suction filter to obtain sodium 2-naphthalenesulfonate. Place the sodium hydroxide in an alkali melting pot, heat (melt) to 290°C, and add sodium 2-naphthalenesulfonate in about 3 hours until the free alkali content is 5% to 6%. After that, it was kept at 320-330°C for 1 hour. Alkali melt is diluted in water and passed SO2 at 70~80℃ until phenolphthalein is colorless. Add water to boil and wash, remove the sodium sulfite, and then dehydrate and distill under reduced pressure to obtain the finished product. The total yield is 73% to 74%. The 2-isopropylnaphthalene method uses naphthalene and propylene as raw materials to produce 2-naphthol while by-product acetone.
Upstream raw material: sodium hydroxide–>sulfuric acid–>nitric acid–>anhydrous sodium sulfite–>sulfur dioxide–>naphthalene–>Congo red test paper–>sodium bisulfite solution–> 2-naphthalenesulfonic acid –>Phenolphthalein–> Sodium 2-naphthalenesulfonate–>Solid caustic soda
Downstream products: 2-naphthylamine–>R-1,1′-bin-2-naphthol–>Pigment Red 21–>2-naphthylamine-1-sulfonic acid–>naphthylamine–>S -1,1′-Bi-2-naphthol–>1-Amino-2-naphthol-4-sulfonic acid–>Acid Blue 74–>2-hydroxy-3-naphthoic acid–>2- Fluoronaphthalene–>6-Methoxy-2-acetnaphthalene–>chrome black T–>6-hydroxynaphthalene-2-boronic acid–>2-naphthol-3,6-disulfonic acid disodium- ->1-Naphthyldiazo-2-hydroxy-4-sulfonic acid inner salt–>2-Amino-8-naphthol-6-sulfonic acid–>Lithol Scarlet–>Pigment Red 53:1 Chemicalbook –>Pigment Orange 5–>Pigment Red 4–>Pigment Red 3–>Mordant Black 17–>Naproxen–>Synthetic Tanning Agent HV–>Neutral Black 2S-RL–>Medium Black BL–>2-Naphthaleneboronic acid–>2-Hydroxy-1-naphthoic acid–>Sodium 6-hydroxy-2-naphthalenesulfonate–>N-phenyl-2-naphthylamine–>Synthesis Tanning agent PNC–>2-naphthylamine-3,6,8-trisulfonic acid–>bis[3-hydroxy-4-[(2-hydroxy-1-naphthyl)azo]-1-naphthalenesulfonate Disodium hydrogen chromate–>Sodium 3-hydroxy-4-[(2-hydroxynaphthalene)azo]-7-nitronaphthalene-1-sulfonate–>Synthetic Tanning Agent No. 9
Post time: Apr-20-2021





