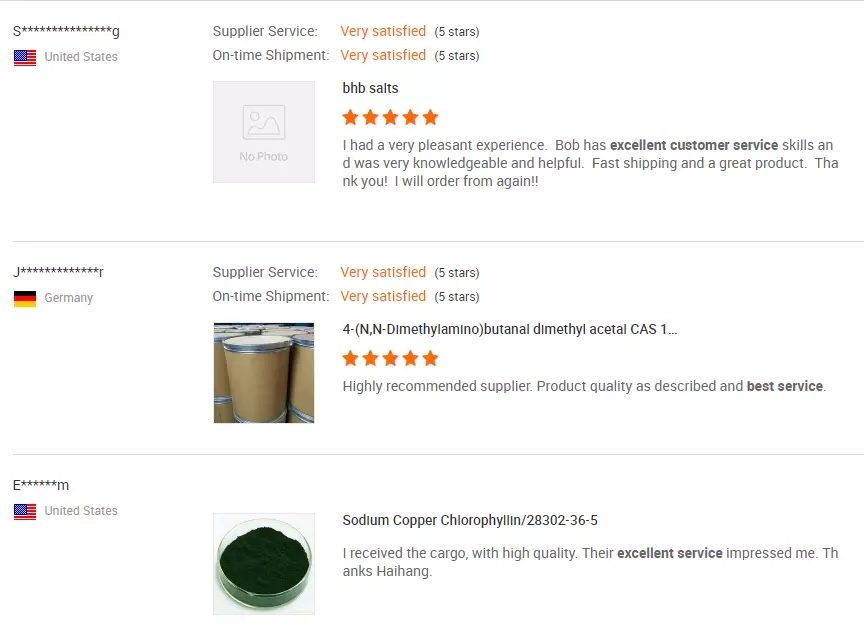
MIT-Ivy Industry is a pharmaceutical and chemical intermediate enterprise integrating production, sales and scientific research.With the advanced production equipment and production technology, we maintain good cooperative relations with scientific research institutions and large and medium universities. We are mainly engaged in the production and sales of a series of pharmaceutical intermediates, especially in the treatment of anti-AIDS, cardiovascular and cerebrovascular drugs, and supporting intermediates of anti-inflammatory drugs.The products of sodium azide, triphenyl chloromethane, L- valine methyl ester hydrochloride are sold at home and abroad.1. Introduction of pharmaceutical intermediatesPharmaceutical intermediates refer to the intermediate products such as raw materials, materials and excipients used in the production of pharmaceutical products.In fact, they are some chemical raw materials or chemical products in the process of drug synthesis.The pictureManufacturers of proprietary drugs and active drug substances need to accept GMP certification. Pharmaceutical intermediates, though used in drug production, are essentially only the synthesis and production of chemical raw materials. They are the most basic and bottom products in the drug production chain and cannot be called drugs, so GMP certification is not required.Pharmaceutical intermediates can be produced in ordinary chemical plants and can be used in the synthesis of medicinal products at certain levels.This also reduces the entry threshold of the industry for intermediate manufacturers.The picture2. Scale of pharmaceutical intermediates industryWith the adjustment of industrial structure, transnational production transfer and further refinement of international division of labor of large multinational pharmaceutical companies, China has become an important intermediate production base in the global division of labor of pharmaceutical industry.According to the Research Report on the Development of China’s Pharmaceutical Intermediates Industry (2016), from 2011 to 2015, China’s pharmaceutical intermediates industry and its total output value increased year by year, with a compound annual growth rate of about 13.5%.Among them, the total output value of pharmaceutical intermediates in China reached 422.56 billion yuan in 2015, an increase of 9.88% year on year.Industry output reached 17.2 million tons, up 10.26 percent year on year.The output value of pharmaceutical intermediates industry in China will be close to one trillion yuan by 2020.The picture3. Industrial characteristics of pharmaceutical intermediatesThe industry is in urgent need of optimization and upgrading: China’s overall technological level is still relatively low, and there are few enterprises producing a large number of advanced pharmaceutical intermediates and supporting intermediates for patented new drugs, which are in the development stage of product structure optimization and upgrading.Only some enterprises with strong research and development strength, advanced production facilities and large-scale production experience can obtain high profits in the competition.Stable business scale: Large-scale manufacturers basically take customized production as their main business model. Under the customized production model, the cooperative relationship between major customers and suppliers is relatively stable, and the closer the cooperation, the higher the degree of trust, and the more cooperation categories provided by major customers will be.It takes a long time to change suppliers. Therefore, as a business with strong stickiness, pharmaceutical intermediates industry enterprises mainly focus on well-known foreign pharmaceutical enterprises at the present stage.Once the company entered the core supplier system of pharmaceutical giants, both the production scale and the gross profit margin maintained a fairly stable state.Low-end export mainly: the main export regions of pharmaceutical intermediates in China are EU, North America, Middle East, Southeast Asia, etc.The export of our country mainly concentrated in vitamin C, penicillin, acetaminophen, citric acid and its salts and esters, such as commodities, the characteristics of the products is products production, production enterprises, the market competition is intense, product price and added value is low, the mass production of them caused the domestic pharmaceutical intermediates market oversupply situation.High-tech products are still mainly imported.Small and medium-sized enterprises: the production enterprises are mostly private enterprises, flexible operation, investment scale is not large, basically between millions to ten or twenty million yuan.Regional concentration: the regional distribution of production enterprises is relatively concentrated, around several major pharmaceutical factories, mainly distributed in Taizhou, Zhejiang and Jintan, Jiangsu as the center of the region.Zhejiang Huangyan, Taizhou, Nanjing Jintan, Shijiazhuang, Jinan (including Zibo), northeast (Siping, Fushun) and other areas with favorable conditions for the development of pharmaceutical intermediates have developed particularly rapidly.Fast product update: a product is generally on the market 3 to 5 years later, its profit rate will drop significantly, which forces enterprises to constantly develop new products or constantly improve the production process, in order to maintain a high production profit.Intensive competition: because the production profit of pharmaceutical intermediates is higher than that of chemical products, and the production process of the two is basically the same, more and more small chemical enterprises join the production of pharmaceutical intermediates, leading to increasingly fierce disorderly competition in the industry.4. Types of pharmaceutical intermediatesThere are many kinds of pharmaceutical intermediates, including cephalosporin intermediates, amino acid protectant series, vitamin intermediates, quinolone intermediates, and other types of intermediates, such as medical disinfectant intermediates, antiepileptic drug intermediates, fluoropyridine intermediates, steroid pharmaceutical intermediates, etc.According to their application fields, they can be divided into antibiotic drug intermediates, antipyretic and analgesic drug intermediates, cardiovascular system drug intermediates, anti-cancer drug intermediates and so on.At present, there are about hundreds of pharmaceutical intermediates products, and constantly innovate, forming many fine molecular industries in the pharmaceutical intermediates industry.There are many types of specific pharmaceutical intermediates.Such as imidazole, furan, phenolic intermediates, aromatic intermediates, pyrrole, pyridine, biochemical reagents, sulfur, nitrogen, halogen compounds, heterocyclic compounds, microcrystalline cellulose, starch, mannitol, lactose, dextrin, ethylene glycol, powdered sugar, inorganic salts, ethanol intermediates, stearic acid, amino acid and ethanol amine salt, sylvite, sodium salt and other intermediates and so on.The picture5. Patent CliffSince 2000, the global generics market has continued to grow faster than the overall pharmaceutical market, growing more than twice as fast as patented drugs.The global generic drug market is expected to reach $180 billion in 2013, and the CAGR of the global generic drug market from 2005 to 2013 is expected to reach 14.7%.The global generics market is expected to grow by 10% to 14% over the next five years, much faster than the expected 4% to 6% growth for the entire pharmaceutical industry.It can be inferred that the development of generic drug market will obviously promote the development of pharmaceutical intermediate industry.From 2010 to 2020, the global pharmaceutical market will usher in a wave of high tide of Patent expiration, among which, from 2013 to 2020, the global Patent expiration varieties will average more than 200 each year, which is known as the “Patent Cliff” in the world.In 2014, there will be a peak of patent drug expiration, in which there will be a peak in 2014, with a total of 326 patent drugs expiring. 2010 and 2017 are the two relative peak years, with 205 and 242 patent drugs expiring respectively. The expired drugs are mainly anti-infective, endocrine, nervous system and cardiovascular drugs, with a huge market size.The large-scale expiration of foreign patented drugs will bring new catalysts to the pharmaceutical intermediates industry in China.Because after the expiration of patent drugs, the production of related generic drugs will explode, which will drive the rapid growth of the demand for related pharmaceutical intermediates.The picture6. Environmental pressureChina is already a major exporter of API intermediates, as well as a major polluter.Pharmaceutical intermediate manufacturers belong to fine chemical industry, there will be corresponding pollution risk.According to statistics from the Ministry of Environmental Protection, the total output value of the domestic pharmaceutical industry is less than 3 percent of the country’s GDP, but the total amount of pollution emissions reaches 6 percent.Among all kinds of drugs, the API mainly represented by vitamins and penicillin belongs to the industry of high pollution and high energy consumption, which pollutes the air and water especially seriously.In accordance with the unified deployment of the Ministry of Environmental Protection, on February 15, 2017, the special inspection team for air quality in the first quarter of 2017 announced that the pressure conduction in Shijiazhuang was not in place, and the county-level government was still mainly based on the Environmental Protection Bureau personnel in the implementation of the heavy pollution weather emergency plan, while other departments were not involved in a high degree.The pollution of small and medium-sized enterprises producing pharmaceutical intermediates in Shijiazhuang is serious.Pharmaceutical enterprises with backward technology will bear high pollution control costs and regulatory pressure, and traditional pharmaceutical enterprises mainly producing high pollution, high energy consumption and low value-added products (such as penicillin, vitamins, etc.) will face accelerated elimination.Adhering to process innovation and developing green pharmaceutical technology has become the future development direction of pharmaceutical intermediates industry.The picture
7. Industry leaders
mit-ivy industry
Zhejiang NHU Company Ltd.Plo Co., Ltd
Lianhe Chemical Technology Co., Ltd.Anhui Bayi Chemical Co. LtdZhejiang Huahai Pharmaceutical Co. LtdZhejiang Hisoar Pharmaceutical Co., Ltd.Jiangsu Jiujiu Technology Co., LtdFederal pharmaceutical (chengdu) co., LTDZhejiang Yongtai Technology Co., Ltd.Suzhou Tianma Specialty Chemicals Co.,Ltd.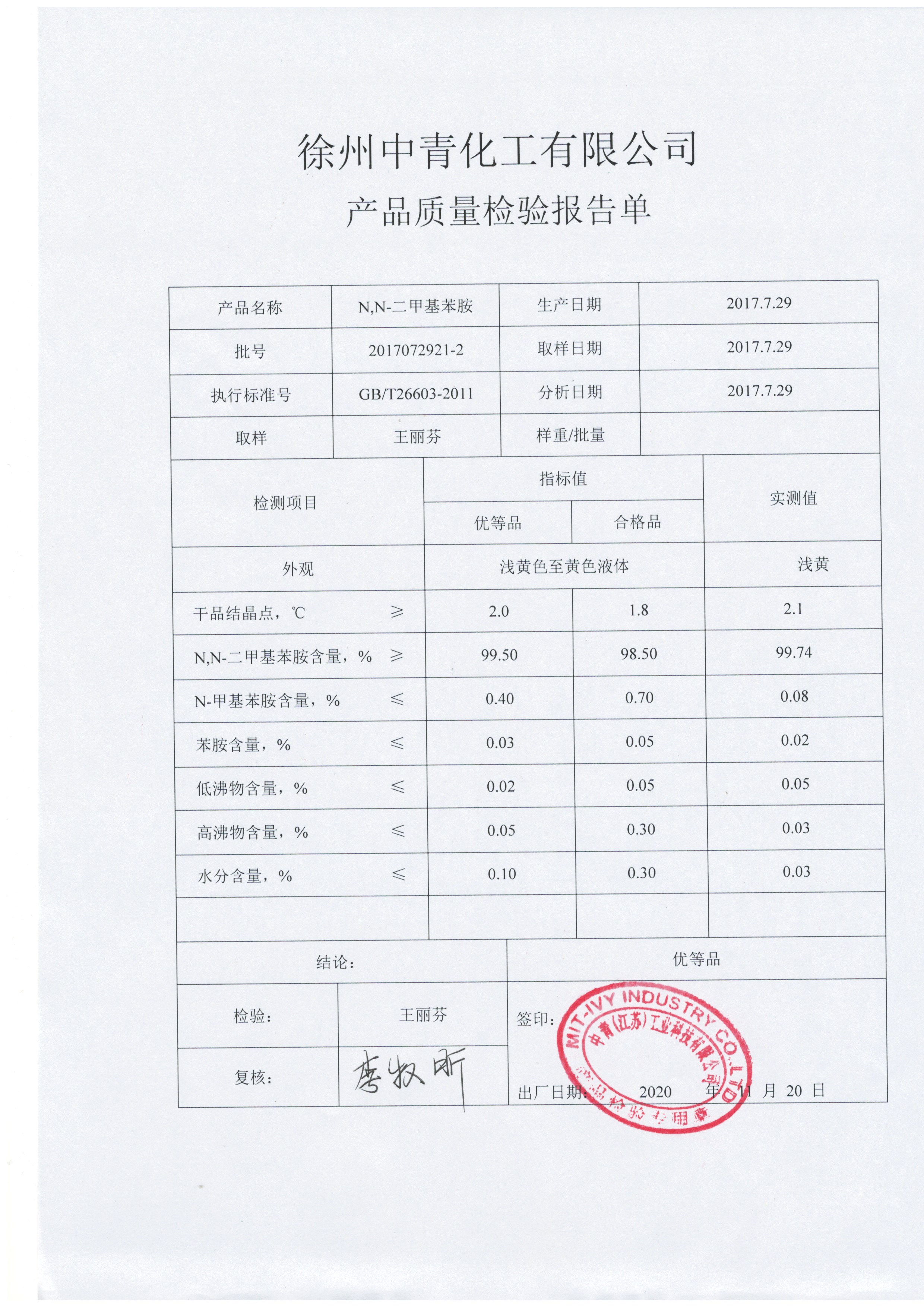
Post time: Apr-12-2021